Iowa Mass Shooting – Are the Police in the Pocket of Big Pharma & the Mental Health Industry?

LinkedIn Posting of Sheriff Adam Infante at Dallas County Sherriff’s Office Mental Health Training, reflecting 2019 suicide data.
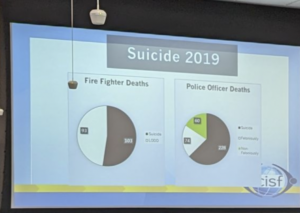
The introduction of behavioral health training for police officers has been hailed as a positive step towards improving law enforcement response to the overwhelming mental health crisis of suicides and mass murders. However, beneath the well-intentioned initiative lies a complex landscape of challenges and unintended consequences that need to be investigated.